Chemical Compatibility Chart
 This Chemical Compatibility Chart is a shorthand tool for describing the suitability of miniature fluidic and pneumatic component materials for use in contact with various chemicals. Use this chart to help guide your component selection.
This Chemical Compatibility Chart is a shorthand tool for describing the suitability of miniature fluidic and pneumatic component materials for use in contact with various chemicals. Use this chart to help guide your component selection.
Use our Chemical Compatibility Chart as guide for evaluating the resistance of polymer and metal materials to a wide range of chemicals found in industrial, commercial, laboratory and medical applications. It can help with the selection of flow control components and tubing samples for evaluation and testing.
An Explanation of the Chemical Compatibility Chart Ratings
- A Excellent - The component material is completely or almost completely inert when used with the specified chemical with negligible effect on mechanical properties.
- B Good - There is some slight chemical attack on the component material that could create slight corrosion or discoloration but otherwise has minor effect on mechanical properties.
- C Fair - The component material is partially attacked or attacked by absorption of the specified chemical at the specified concentration and temperature levels and swelling may occur. The life expectancy of the component will be shortened and it is recommended that a different component material be used in these cases.
- D Poor - The component material has no resistance to chemical attack by the specified chemical at the specified concentration and temperature and immediate damage may occur. Severe effects make this combination unsuitable for ANY use.
- N/A Unknown - There is little or no technical information available for this combination of component material and chemical at this temperature and concentration. This combination has no recommendation and may require extensive testing and evaluation for its safe use.
How to use this chart to determine compatibility
Find the name of the chemical along the left column of the chart. The chemicals are listed alphabetically.
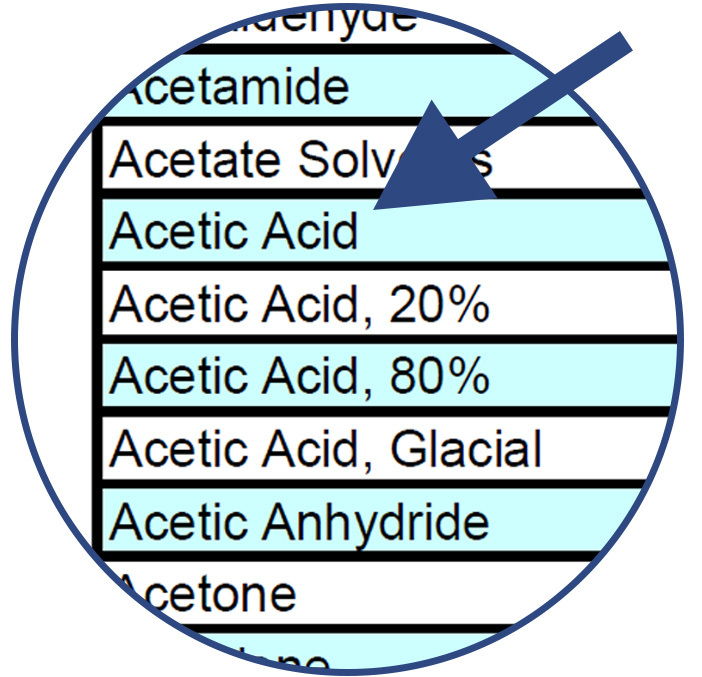
Compare the chemical with the various component material options listed along the top row of the chart.
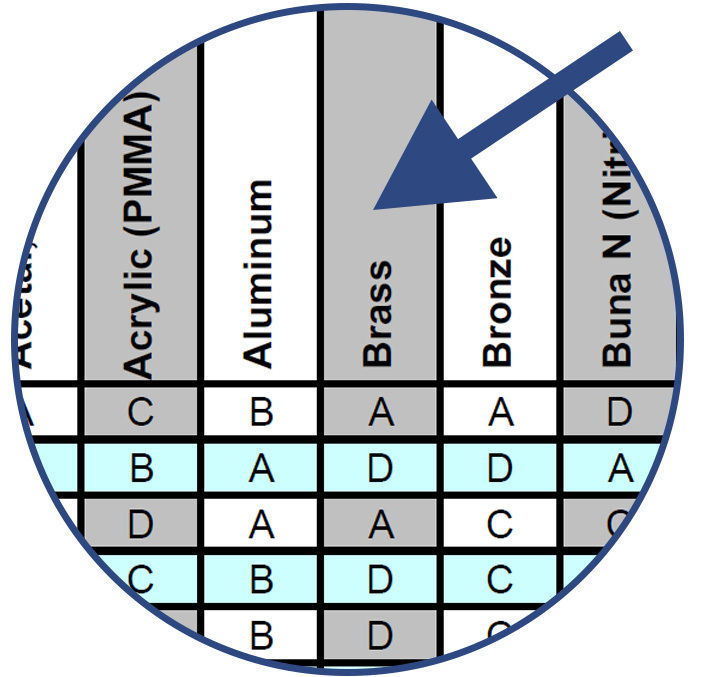
Note the compatibility rating of the combination of chemical and component material and choose the best materials for sampling, evaluation and testing.
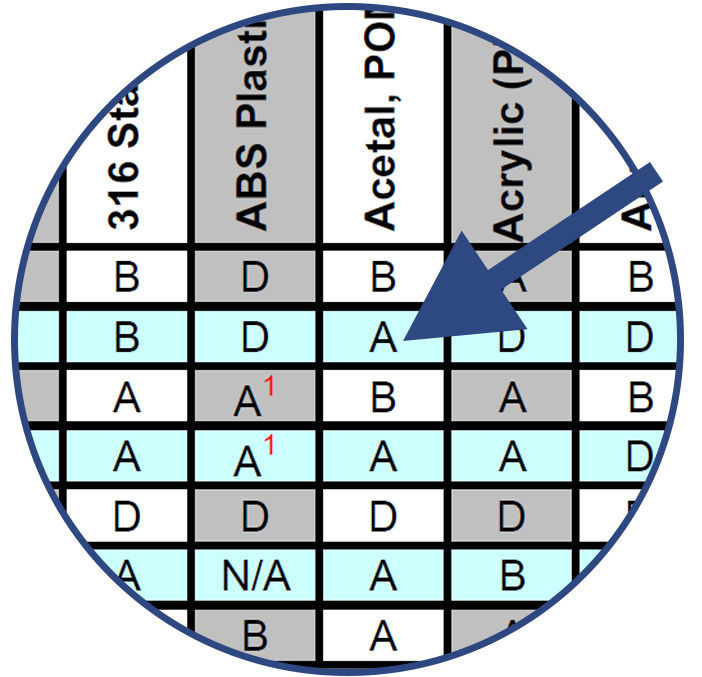
Combinations of Chemicals
Chemical compatibility of component parts is extremely important because of possible rapid degradation and failure if the materials are not compatible with the environment where they are used or the chemical they are in contact with. Chemical compatibility is often compromised when handling more than one chemical or when handling compounds containing impurities. This listing does not provide information on chemical combinations.
Temperature Considerations
Resistance to chemicals is a function both of temperature and concentration. There are many reagents which can be safely handled only below a certain temperature. It is important to note all temperature references within this compatibility chart. All information provided in this chart is based on an assumed ambient temperature of about 70 °F (21 °C) unless otherwise noted. Mixing or dilution of certain chemicals as well as combinations of mixing and dilution of chemicals may cause undesirable chemical reactions including the production of heat which in turn can cause component failures.
Plastic Components
Chemicals can affect the strength, flexibility, surface appearance, color, dimensions or weight of plastic components and component parts. The sorts of interactions that can take place between a chemical and a plastic are of three basic types: chemical attack on the plastic, stress cracking and physical change of the plastic such as softening and swelling.
Stress Cracking
Environmental stress cracking is the failure of a thermoplastic component when exposed to certain types of chemicals. This failure is not purely a result of chemical attack. A combination of three factors cause stress cracking: tensile stress, a stress cracking chemical and the inherent susceptibility of a thermoplastic to stress cracking. Stress cracking chemicals include detergents, surfactants, lubricants, oils, ultra-pure water and plating additives such as brighteners and wetting agents.
Relatively small concentrations of stress cracking agent may be sufficient to cause cracking. Chemicals that do not normally affect the properties of an unstressed thermoplastic may cause stress cracking under thermal or mechanical stress such as constant internal pressure or frequent thermal or mechanical stress cycles.
Interpretation of Chemical Resistance
The information in this chart has been supplied by reputable sources believed to be reliable and accurate. This information is to be used ONLY as a guide to selecting components for testing for appropriate chemical compatibility. Variations in temperature, concentrations, chemical combinations, durations of exposure and other factors may affect the performance of the component. Ultimately, the user must determine the suitability of the materials used. Before permanent installation, test the component with the chemicals and under the specific environmental conditions of your application. ISM does not warrant, neither express nor implied, that the information in this chart is accurate or complete or that any material is suitable for any purpose. It should be noted in the chart that the "A" rating does not mean or imply that the plastic material will perform within the original specifications.
Variations in chemical behavior during handling due to factors such as temperature, pressure, and concentration can cause equipment to fail even though it passed an initial test. Use suitable guards and personal protections when handling chemicals.
Chemicals and chemistry resources
Ethanol or ethyl alcohol
Alcohols
Alcohols are organic compounds with a hydroxyl group attached to one end of the molecule. Hydroxyl groups are one hydrogen atom bonded or attached to an oxygen atom.
Get more information at the Alcohol page from Encyclopædia Britannica.
Alkalis
Alkalis are a subset of bases. Chemically, alkalis are soluble hydroxides (OH−) or carbonates of alkali metals: lithium, sodium, potassium, rubidium and cesium. Like other bases, alkalis react with and neutralize acids. Generally, alkalis tend to be strong bases and are considered caustic. That means they will react chemically with organic materials and burn, corrode or destroy them.
Get more information at the Alkali page from Encyclopædia Britannica.
Ethylene
Aliphatic hydrocarbons
Methane, acetylene, and ethylene for example. Aliphatic hydrocarbons are organic compounds whose atoms do not link together to form a ring.
Get more information at the Aliphatic Hydrocarbon Definition page from ThoughtCo.
Aromatic hydrocarbons
Benzene is an aromatic hydrocarbon. These chemicals are organic compounds that contain one or more benzene rings. A benzene ring is a hexagonal ring of six carbon atoms.
Get more information at the Aromatic Hydrocarbon page from Wikipedia.
Bases
Bases are the opposite of acids. Like acids, bases also tend to be very corrosive depending on the specific chemical and how concentrated it is. Lye is a strong base that most people are familiar with.
Get more information at the Base page from Encyclopædia Britannica.
Vinyl chloride
Chlorinated hydrocarbons
Chlorinated hydrocarbons are a group of organic chemicals composed of carbon, chlorine, and hydrogen. Vinyl chloride, chloromethane and DDT are some of the more familiar examples of chlorinated hydrocarbons. This group of chemicals sometimes occur naturally but the majority are synthesized. Chlorinated hydrocarbons have many industrial applications. These include uses as solvents, pesticides, coatings, synthetic rubbers. A large variety of them are used as ingredients for synthesizing other chemicals and materials.
Get more information at the Organochloride page at the Wikiwand website.
Detergents
Detergents are considered surfactants. Surfactants or surface-active agents are compounds that, in solution, reduce the surface tension of a solvent. This increases the tendency of the solvent to spread and cover any surfaces it is in contact with. Surfactants are partly hydrophilic (water soluble) and party soluble in oils. This allows them to act as emulsifying agents and this makes them useful as cleaners. Detergents can loosen dirt from surfaces and hold it in suspension so it can then be rinsed away. Detergents are produced by a different chemical process than soaps.
Get more information at the Detergents and Soaps page of the ExplainThatStuff website.
Esters
Esters are a class of organic compounds with a particular chemical structure and makeup. They generally react with water to produce alcohols and organic or inorganic acids. One characteristic of esters is that they tend to smell fruity or sweet. Commercially, esters are widely used as solvents for plastics, plasticizers, resins and lacquers. They are also very important for the manufacture of synthetic lubricants.
Get more information at the Esters page of the Introduction to Chemistry, Organic Chemistry section at the Lumen Learning website.
Methylene chloride
Halogenated solvents
Chloroform, carbon tetrachloride, and methylene chloride are examples of halogenated solvents. These solvents are compounds that contain halogen atoms (see below).
Get more information at the Halogenated Solvents page from the LibreTexts™ Chemistry library.
Halogens
Halogens are the group non-metallic elements that includes fluorine, chlorine, bromine and iodine. These elements are poisonous and are highly chemically reactive.
Get more information at the Halogen page from Wikipedia.
Acetone
Ketones
Ketones are a class of organic compounds that have a particular chemical structure and makeup. The best known and simplest ketone is the industrial solvent acetone. Ketones are widely found in nature. There are also a large number of ketones that are used in industrial and in biopharmaceutical processes.
Get more information at the Aldehydes and Ketones page at the Michigan State University Department of Chemistry website.
Mineral acids
Sulfuric acid, hydrochloric acid and nitric acid for example. Mineral acids are acids made from an inorganic compound. These acids easily dissolve in water and are very acidic.
Get more information at the What is a Mineral Acid page from ThoughtCo.
Sulfuric acid
Organic acids
Carboxylic acids and sulfonic acids are organic acids. These acids are organic compounds that have acidic properties: liquids, corrosive and so on. Organic acids tend to be weak acids and do not dissolve completely in water.
Get more information at the Organic Acid page from Wikipedia.
Oxidizing environments
Oxygen, hydrogen peroxide and the halogens are examples of oxidizers. In the Earth's atmosphere, iron is converted to iron oxide or rust because there is plenty of oxygen available.
Get more information at the Oxidizing Agent page from Wikipedia.
